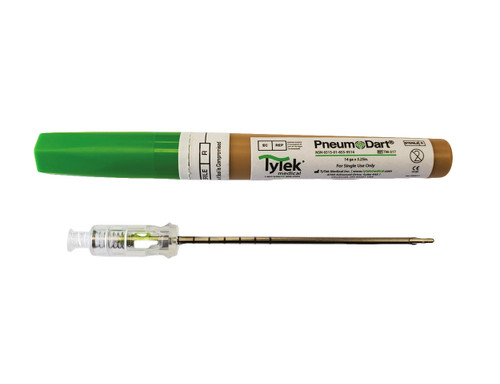
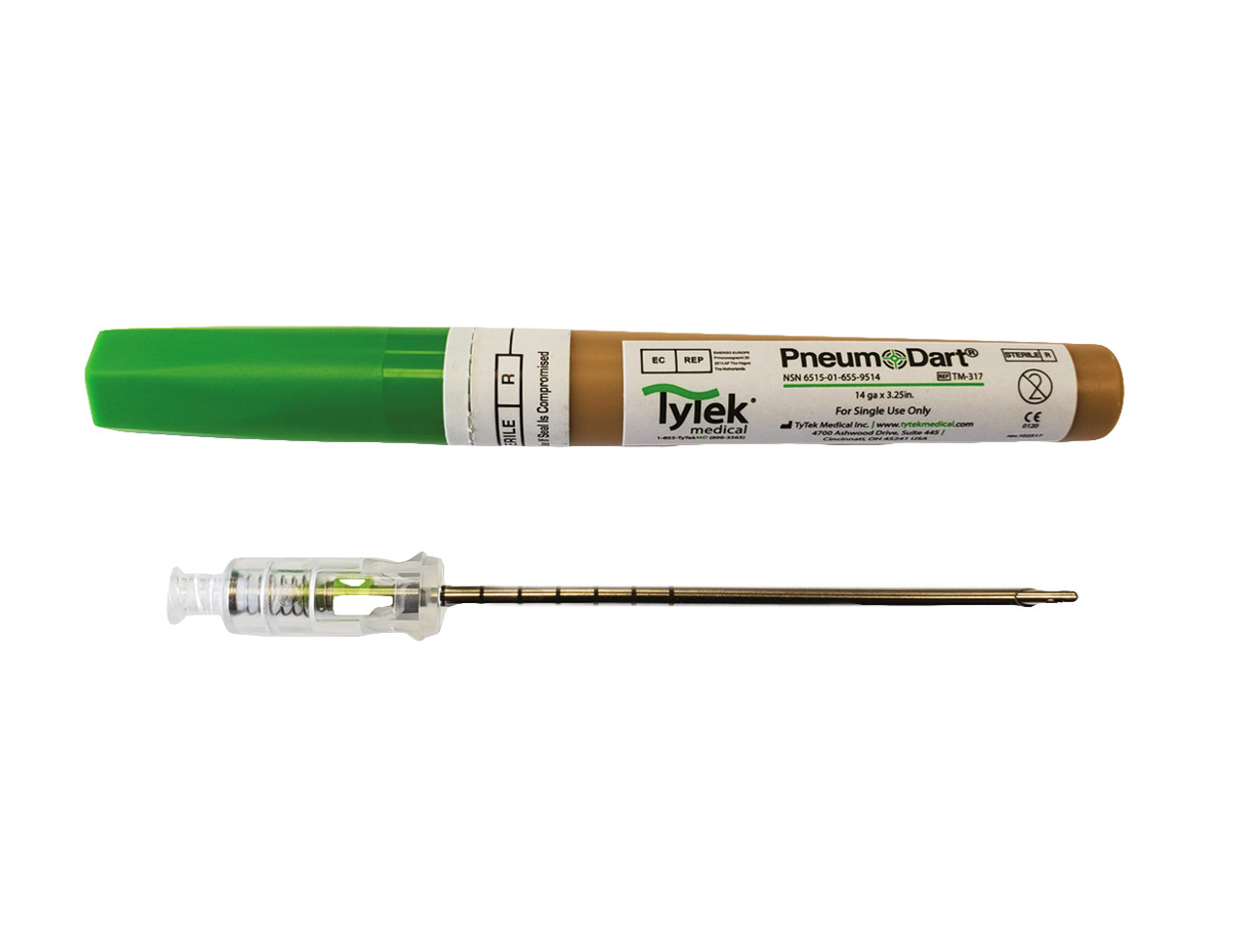
PneumoDart Tension Pneumothorax Chest Needle Decompression
- Weight:
- 52.00 Grams
- Shipping:
- Eligible for free shipping!
- 14 gauge x 3.25”
- Veress safety tip to reduce the risk of puncturing internal organs when inserted.
- Visible cap head to monitor air or blood leaving the chest.
- Luer lock cap allows for an active drawing or elimination of air or blood from the chest.
- NSN 6515-01-655-9514 and CE marked.
The PneumoDart is a reliable solution for treating a tension pneumothorax injury, or sucking chest wound.
It offers better efficiency, safety and important physical features. Typical methods for treating this kind of injury include the application of a plastic seal over the chest wound to inserting a needle catheter into the chest to drain air out of the chest to treat collapsing lung and relieve pressure. However, successful application of a traditional pneumothorax needle has proven difficult.
The PneumoDart needle is 3.25” which is the optimal length as recommended by the Tactical Combat Casualty Care (TCCC) who inspired the PneumoDart and developed it with the US Department of Defense during a robust testing and evaluation phase. The PneumoDart innovation helps you to get it right, first time, where successful decompression with traditional decompression needles can prove difficult. It offers efficiency, safety and important physical features.
The needle was developed with the US Department of Defense during a robust testing and evaluation phase.
Instructions For Trained Medical Personnel
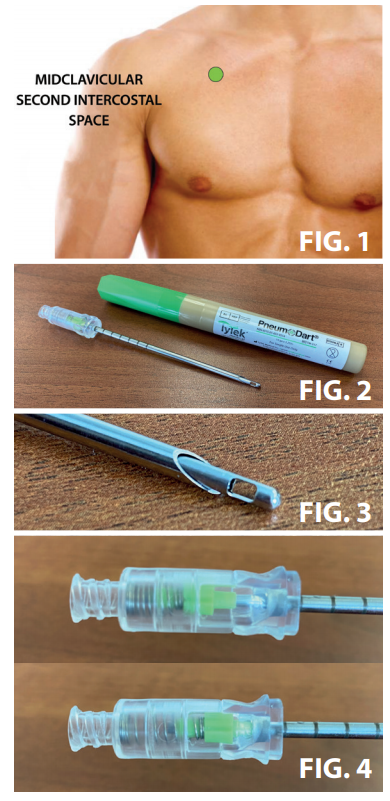
• Select the location for the needle to enter by identifying the second intercostal space on the anterior chest at the mid-clavicular line on the same side of the body as the injury. (FIG. 1)
• Use antimicrobial solution to cleanse the needle-insertion site.
• Inspect that the safety seal is intact. If not intact, do not use the product. With a firm grip on the PneumoDart®, twist the hex cap to break the seal, and remove the cap.
• Remove the PneumoDart® needle from its case.
• Insert the needle assembly into the patient’s skin over the superior border of the third rib at the mid-clavicular line. Direct the needle into the intercostal space at a 90º angle to the chest wall, being careful that the entry is not medial to the nipple line and not directed toward the heart.
• Spring-loaded, retractable safety tip (FIG 3) retracts as the needle is pushed through the chest wall and automatically advances once the pleural cavity is penetrated, if a pneumothorax is present.
• Visual Needle Position Indicator (FIG 4). Once the PneumoDart® penetrates pleural cavity, the green indicator will click in the down position and you will hear a sudden escape of air as the tension pneumothorax is decompressed.
• Secure in a manner directed by your training.
• Monitor the patient carefully for any tension pneumothorax recurrences or respiratory distress.
Body Substance Isolation Alert
Always use appropriate body substance isolation procedures and personal protective equipment in order to prevent contact with contaminated fluids.
Caution
PneumoDart® should only be used by personnel who have received proper training on procedures for relieving a tension pneumothorax with this device. Improper use of PneumoDart® could cause injury. Use PneumoDart® as directed by your EMS authority or by a physician. Do not place PneumoDart during active chest compressions.
Potential hazards of needle decompression include cardiac tamponade, life-threatening bleeding due to pulmonary artery, aorta or intercostal vessel injury, non-therapeutic insertion and potential nerve injury at insertion site. Hazards can be avoided by adhering to approved protocols, training and site placement. Failure to adhere may result in patient decompensation, injury and/or death.
Disposal
PneumoDart® is for single-use only. It is not re-usable under any circumstances. It may be used once, and then must be properly disposed. Dispose of PneumoDart® in a way that assures body substance isolation of potentially infectious substances and prevents sharps injury.
Storage
Keep PneumoDart® dry, stored in temperatures between 10°F and 140°F.
Limited Warranty
The products and components contained in this package are medical devices intended solely for use by appropriately trained healthcare professionals. TyTek Medical Inc. warrants that the products and components are free from defects in material and workmanship and fit soley for use as medical devices for the specific purpose described in the manual by trained healthcare professionals for a period of 12 months from the date of shipment by TyTek Medical Inc. Any use of the products or components beyond the specific use described in this manual or by a person not trained or legally authorized to use the products or components voids this limited warranty.
EXCEPT AS SET FORTH HEREIN, TYTEK MEDICAL INC. MAKES NO OTHER WARRANTIES, EXPRESS OR IMPLIED, REGARDING THE PRODUCTS OR COMPONENTS AND SPECIFICALLY DISCLAIMS THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE.